Comprehensive Pharmacovigilance Solution
Comprehensive Drug Safety Monitoring: Track, analyze, and manage drug safety data with precision.
Regulatory Compliance: Stay aligned with global pharmacovigilance standards.
Real-time Reporting: Get instant alerts for adverse drug reactions (ADRs).
Customizable & Scalable: Adapt the software to your organization's needs.
Who Can Benefit from Our Pharmacovigilance Software?
With our 15+ years of experience, we have covered ample industries by fulfilling each tailored needs of clients.
-

Pharmaceutical Industry
-
Healthcare Providers (Hospitals & Clinics)
-

Regulatory Agencies
-

Clinical Research & Trials
-

Biotechnology & Life Sciences Companies
-

Contract Research Organizations (CROs)
-

Insurance & Health Policy Organizations
-

Medical Device Companies
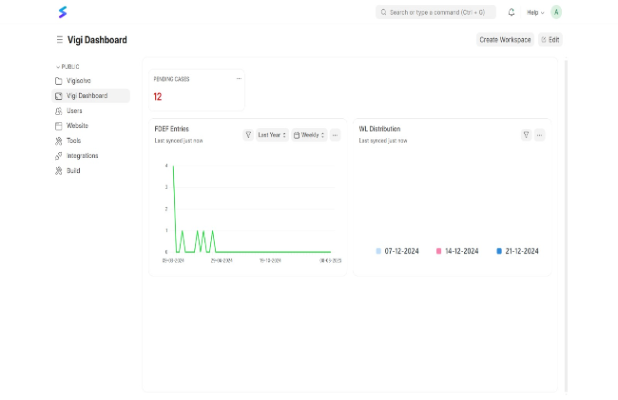
Explore the VigiSolvo Software Features
Pharmacovigilance software ensures seamless drug safety management by efficiently tracking adverse events, automating regulatory reporting and streamlining compliance processes.
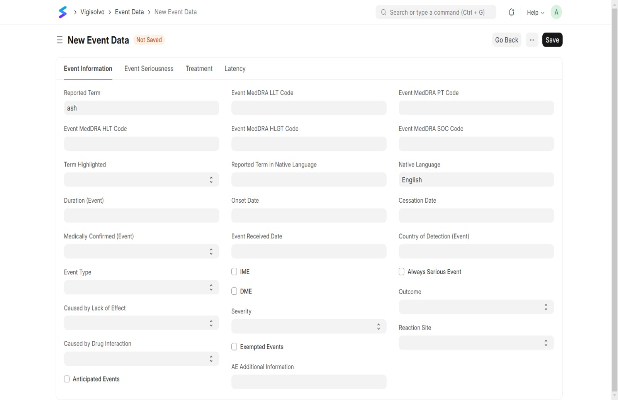
Ensure accurate reporting with a user-friendly interface, customizable forms, and seamless EHR/CTMS integration. Automate data capture, reduce errors, and enhance compliance.
User-Friendly Interface
Customizable Forms
Seamless Data Integration
Leverage advanced analytics, risk assessment tools, and real-time monitoring to detect safety signals early, prioritize investigations, and implement mitigation strategies.
Advanced Analytics
Risk Assessment Tools
Real-Time Monitoring
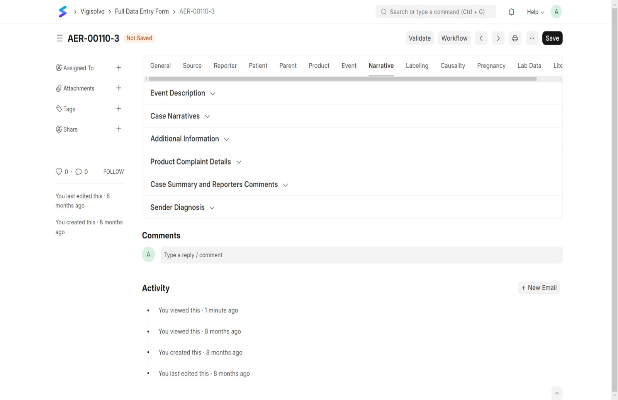
Develop and adapt risk mitigation plans with REMS programs, dynamic safety profile management, and real-time risk assessment to ensure patient safety and regulatory compliance.
Risk Evaluation and Mitigation Strategies (REMS)
Opportunity TrackingSafety Profile Management
Adaptive Risk Management
Automate regulatory reporting, maintain audit trails for transparency, and monitor compliance with a real-time dashboard to swiftly address any non-conformities.
Automated Regulatory Reporting
Audit Trail Documentation
Compliance Monitoring Dashboard
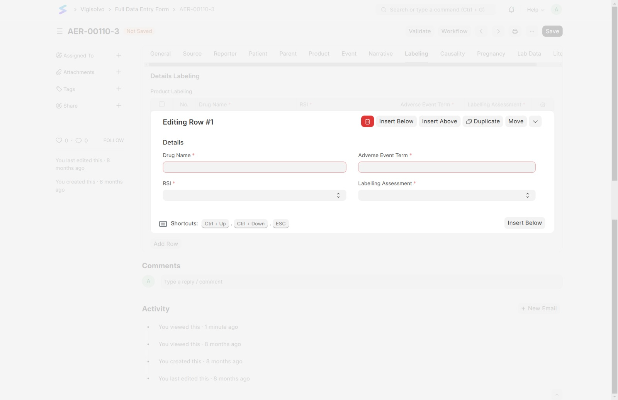

Generate tailored safety reports, visualize data trends through interactive dashboards, and forecast risks with trend analysis for proactive decision-making.
Customizable Reporting Templates
Interactive Data Visualization
Trend Analysis and Forecasting
Seamlessly integrate with CTMS, EHRs, and regulatory systems to automate reporting, enhance interoperability, and analyze large datasets for better risk assessment.
System Interoperability
Regulatory Reporting Systems Integration
Data Analytics Platform Integration
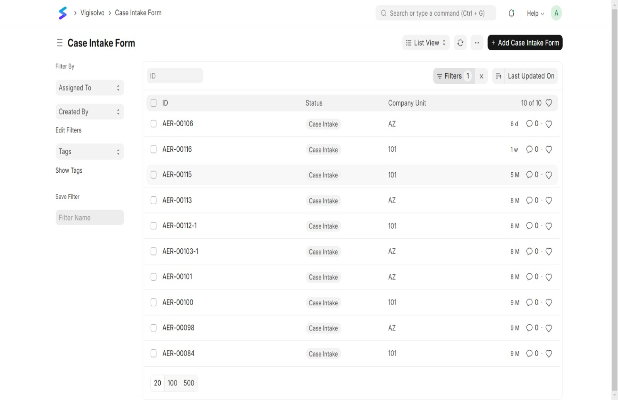

Ensure accurate reporting with a user-friendly interface, customizable forms, and seamless EHR/CTMS integration. Automate data capture, reduce errors, and enhance compliance.
User-Friendly Interface
Customizable Forms
Seamless Data Integration

Leverage advanced analytics, risk assessment tools, and real-time monitoring to detect safety signals early, prioritize investigations, and implement mitigation strategies.
Advanced Analytics
Risk Assessment Tools
Real-Time Monitoring

Develop and adapt risk mitigation plans with REMS programs, dynamic safety profile management, and real-time risk assessment to ensure patient safety and regulatory compliance.
Risk Evaluation and Mitigation Strategies (REMS)
Opportunity TrackingSafety Profile Management
Adaptive Risk Management

Automate regulatory reporting, maintain audit trails for transparency, and monitor compliance with a real-time dashboard to swiftly address any non-conformities.
Automated Regulatory Reporting
Audit Trail Documentation
Compliance Monitoring Dashboard

Generate tailored safety reports, visualize data trends through interactive dashboards, and forecast risks with trend analysis for proactive decision-making.
Customizable Reporting Templates
Interactive Data Visualization
Trend Analysis and Forecasting

Seamlessly integrate with CTMS, EHRs, and regulatory systems to automate reporting, enhance interoperability, and analyze large datasets for better risk assessment.
System Interoperability
Regulatory Reporting Systems Integration
Data Analytics Platform Integration
Why To Choose Vigisolvo ?
Scalable & Flexible
Modular design allows easy addition or removal of features as your organization grows.
Seamless Integration
Ensures smooth data access and interoperability with other systems.

User-Friendly Interface
Intuitive navigation reduces the learning curve and boosts productivity.

Customizable Dashboards
Enables professionals to prioritize tasks efficiently.

Regulatory Compliance
Regular updates align with the latest industry standards.

Dedicated Support
Comprehensive training during onboarding and continuous assistance.
